Validation
Validation
We provide practical services with validation based on GMP.
Since pharmaceutical products are directly related to human health and life, strict quality control is required from the manufacturing process to the final product. The GMP ordinance of the Ministry of Health, Labor and Welfare and other regulations require the validation (proper verification of manufacturing and quality control methods) in order to manufacture high-quality pharmaceutical products that ensure efficacy and safety. We provide our system integration for manufacturing and production control to comply with the validation.
We provide a wide range of services responding to our clients' needs, including CSV (Computerized System Validation) consulting, DQ (Design Quality Validation), IQ (Installation Quality Validation), OQ (Operation Quality Validation), operational support, and calibration SOP (Standard Operating Procedures) creation.
We offer a variety of plans to meet requirements for new installation and facility restoration.
Approaching Validation.
We have been engaged in the validation of various manufacturing facilities including pharmaceutical substances, investigational drugs, and formulation-related facilities and equipment for more than 40 years since GMP was first established in Japan.
| 1980 | Joined the International Society for Pharmaceutical Engineering (ISPE) at the time of its establishment. |
|---|---|
| 1983 | Received an order for a vitamin synthesis facility control system from a certain company, and became a pioneer in our pharmaceutical field. |
| 1987 | Established a coating equipment system for a formulation plant. |
| 1990 | Established an automation and networking system for pharmaceutical manufacturing machinery. |
| 1992 | Participated in the committee for the development of the "Manual for Proper Computer Management" (former Ministry of Health and Welfare) |
| 1994 | Attended validation-related seminars and a GMP consulting seminar at Kemper Masterson (KMI) in the U.S. |
| 1995 | Held Validation Roundtable in Tokyo by Rockwell, KMI, and SANKO Computer Software Pharmaceutical companies, pharmaceutical machinery manufacturers, engineering companies, etc. (KMI: now PAREXEL International) |
| 1997-1998 | Participated in consulting for VE plant equipment control at a pharmaceutical plant in the U.S. |
| 2001 | Participated in 21 CFR Part 11 opinion exchange meetings (U.S.A.) Participated in meetings with Rockwell, Brock Solution, etc. (U.S.A.) |
| 2003 | Participated in 21 CFR Part 11 opinion exchange meetings (U.S.A.) Participated in meetings with Rockwell, Brock Solution, etc. (U.S.A.) |
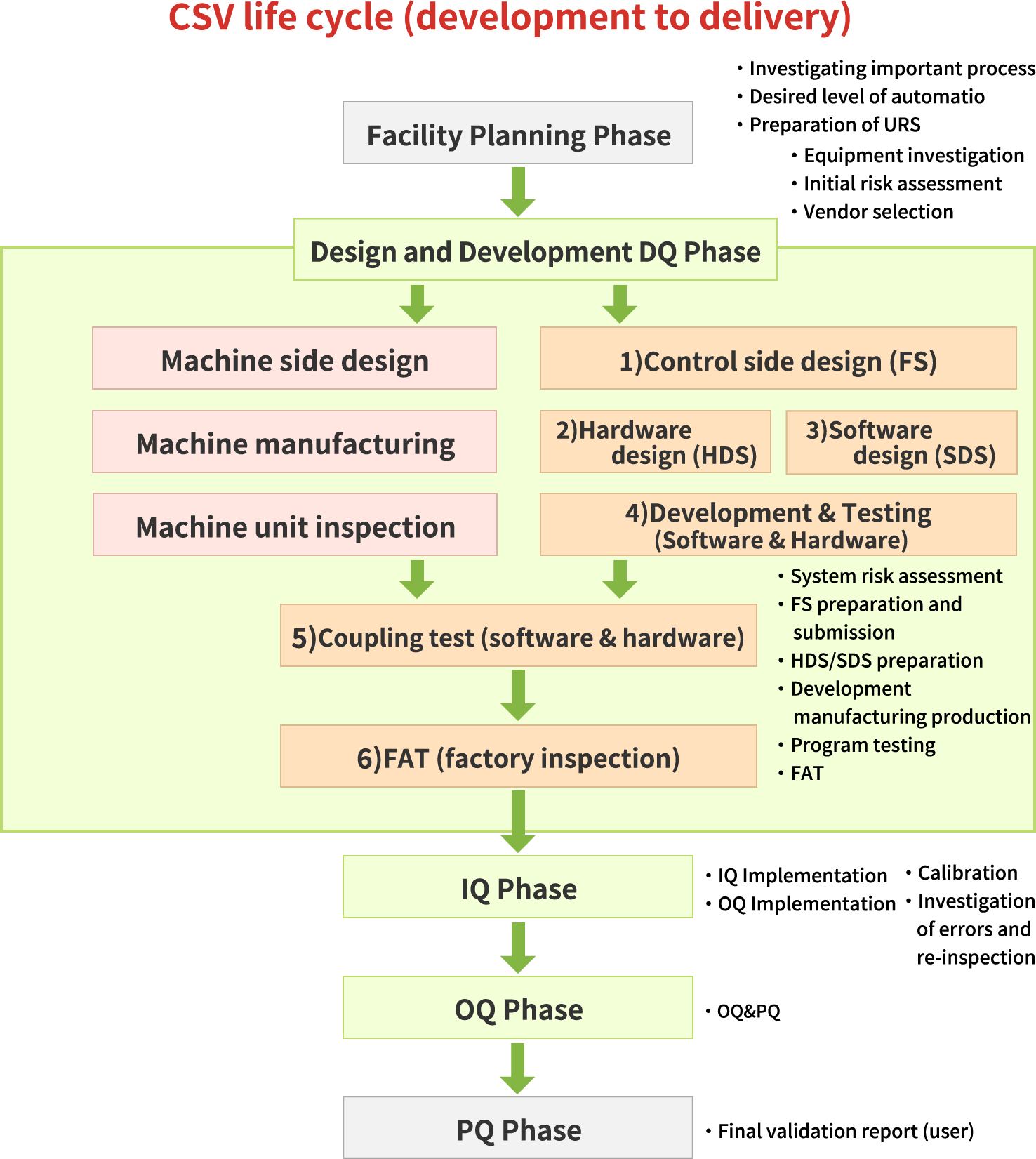
Contents of CSV eligibility check
The following are the main contents of DQ, IQ, and OQ among the operations with the computerized system appropriate management guidelines.
DQ Design Qualification

- URS (User Requirement Specification) preparation support
- Risk assessment
- FS (Functional Specification) preparation
- DS (Design Specification) preparation
System testing, FAT, SAT

IQ installation qualification

- IQ implementation (user factory work)
OQ operation qualification

- Plan preparation
- OQ (user factory work)
Other Support Services
Development Planning Support / PQ (Performance Qualification) Support / System Operation and Management Support / System Retirement Planning Support
Please contact us for more detail >>>